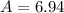Lithium-6 has a mass of 6.0151 amu and lithium-7 has a mass of 7.0160 amu. the relative abundance of li-6 is 7.42% and the relative abundance of li-7 is 92.58%. based on this data alone, calculate the average atomic mass for lithium to the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Lithium-6 has a mass of 6.0151 amu and lithium-7 has a mass of 7.0160 amu. the relative abundance of...
Questions

Business, 24.06.2019 04:30



Biology, 24.06.2019 04:30

Mathematics, 24.06.2019 04:30


Mathematics, 24.06.2019 04:30

History, 24.06.2019 04:30


History, 24.06.2019 04:30

English, 24.06.2019 04:30



Mathematics, 24.06.2019 04:30

History, 24.06.2019 04:30


History, 24.06.2019 04:30

Mathematics, 24.06.2019 04:30

Mathematics, 24.06.2019 04:30

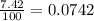
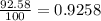
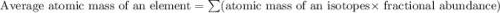
![A=\sum[(6.0151\times 0.0742)+(7.0160\times 0.9258)]](/tpl/images/0113/1342/3dcbf.png)