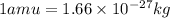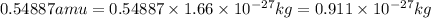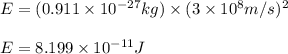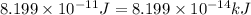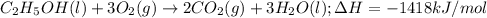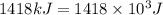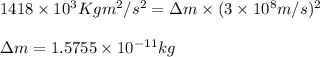3. when two atoms of 2h (deuterium) are fused to form one atom of 4he (helium), the total energy evolved is 3.83 × 10-12 joules. what is the total change in mass (in kilograms) for this reaction? 4. the mass of a proton is 1.00728 atomic mass units (amu) and the mass of a neutron is 60co nucleus whose nuclear mass is 1.00867 amu. what is the mass defect (in amu) of a 27 59.9338 amu? what is the mass defect in kilograms? what is the energy equivalent of this mass in kilojoules? 5. the equation shows one mole of ethanol fuel being burned in oxygen. convert the energy released into its equivalent mass. c2h5oh(l) + 3 o2(g) 2 co2(g) + 3 h2o (l) δh = -1418 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1


Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
3. when two atoms of 2h (deuterium) are fused to form one atom of 4he (helium), the total energy evo...
Questions

Arts, 08.10.2021 17:20

Chemistry, 08.10.2021 17:20

Mathematics, 08.10.2021 17:20



Mathematics, 08.10.2021 17:20


Arts, 08.10.2021 17:20

Mathematics, 08.10.2021 17:20

Mathematics, 08.10.2021 17:20



Biology, 08.10.2021 17:20

History, 08.10.2021 17:20

Mathematics, 08.10.2021 17:20

Chemistry, 08.10.2021 17:20


Mathematics, 08.10.2021 17:20

Biology, 08.10.2021 17:20

Health, 08.10.2021 17:20

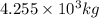
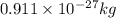 and energy equivalent to this mass is
and energy equivalent to this mass is 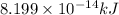
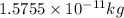

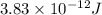
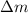 = mass change = ?
= mass change = ?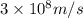
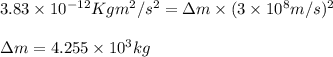
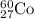
![\Delta m=[(n_p\times m_p)+(n_n\times m_n)+]-M](/tpl/images/0113/2964/f34f4.png)
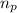 = number of protons = 27
= number of protons = 27
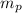 = mass of one proton = 1.00728 amu
= mass of one proton = 1.00728 amu
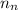 = number of neutrons = 33
= number of neutrons = 33
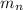 = mass of one neutron = 1.00867 amu
= mass of one neutron = 1.00867 amu
![\Delta m=[(27\times 1.00728)+(33\times 1.00867)]-[59.9338]\\\\\Delta m=0.54887amu](/tpl/images/0113/2964/c0b92.png)