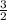Chemistry, 20.07.2019 23:30 jeffreyaxtell4542
One way of obtaining pure sodium carbonate is through the decomposition of the mineral trona, na3(co3)(hco3)·2h2o. 2na3(co3)(hco3)·2h2o(s) → 3na2co3(s) + co2( g. + 5h2o( g. when 1.00 metric ton (1.00 × 103 kg) of trona is decomposed, 0.650 metric ton of na2co3 is recovered. what is the percent yield of this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1


Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1
You know the right answer?
One way of obtaining pure sodium carbonate is through the decomposition of the mineral trona, na3(co...
Questions

Physics, 06.04.2021 03:50

Mathematics, 06.04.2021 03:50


Physics, 06.04.2021 03:50



English, 06.04.2021 03:50

Mathematics, 06.04.2021 03:50

Chemistry, 06.04.2021 03:50

History, 06.04.2021 03:50

Mathematics, 06.04.2021 03:50

Mathematics, 06.04.2021 03:50

English, 06.04.2021 03:50



Mathematics, 06.04.2021 03:50

History, 06.04.2021 03:50

Chemistry, 06.04.2021 03:50