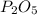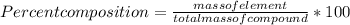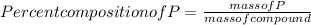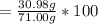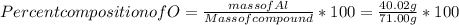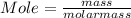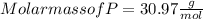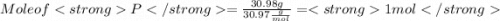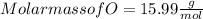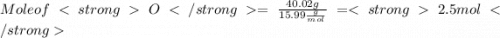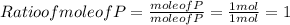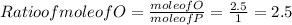Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1
You know the right answer?
When a 30.98-g sample of phosphorus reacts with oxygen, a 71.00-g sample of phosphorus oxide is form...
Questions

English, 12.04.2020 21:16

Chemistry, 12.04.2020 21:16




Mathematics, 12.04.2020 21:17



Mathematics, 12.04.2020 21:17

Mathematics, 12.04.2020 21:17

Mathematics, 12.04.2020 21:18


Social Studies, 12.04.2020 21:18

English, 12.04.2020 21:18


English, 12.04.2020 21:19



Mathematics, 12.04.2020 21:19