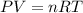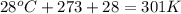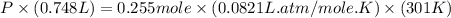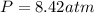Chemistry, 21.07.2019 04:00 robertstoll81
Question 1 a sample of 0.255 mole of gas has a volume of 748 ml at 28°c. calculate the pressure of this gas. (r= 0.0821 l ∙ atm / mol ∙ k) 0.784 atm 8.42 atm 0.00842 atm 7.84 × 10-4 atm none of the above

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2


You know the right answer?
Question 1 a sample of 0.255 mole of gas has a volume of 748 ml at 28°c. calculate the pressure of t...
Questions


Physics, 03.12.2020 23:30



History, 03.12.2020 23:30

Mathematics, 03.12.2020 23:30

Business, 03.12.2020 23:30

History, 03.12.2020 23:30


Biology, 03.12.2020 23:30



History, 03.12.2020 23:30




Mathematics, 03.12.2020 23:30

Business, 03.12.2020 23:30