5
789
10 11
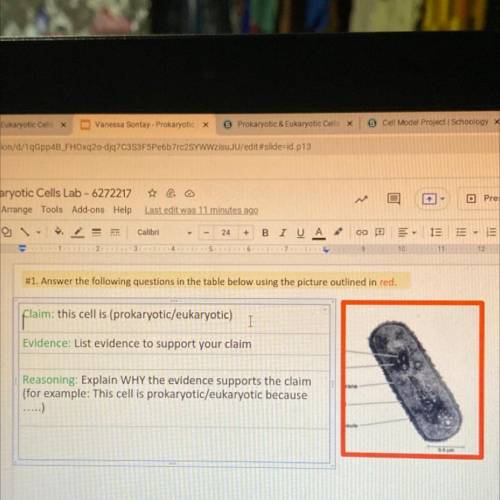
Pili
Nucleoid
rax
Ribosomes
+
Plasma membran...

Biology, 25.09.2021 03:00 spiritedawayoy6378
5
789
10 11
Pili
Nucleoid
rax
Ribosomes
+
Plasma membrane
Cell wall
ران
Capsule
Flagella
(b) A thin section through the
bacterium Bacillus coagulans
(TEM)
(a) A typical
rod-shaped
hacterium

Answers: 1


Another question on Biology

Biology, 21.06.2019 16:30
Melissa lives in a town called greendale. she discovers that when she lights a match and holds it near her kitchen faucet, the little flame grows into a larger fire. melissa is surprised because she thought the water would extinguish the flame rather than ignite it further. her mother tells her that the water might be contaminated with dissolved methane, a primary component in natural gas. melissa investigates the matter and finds that cases of methane contamination started shortly after a company began fracking for natural gas in the area. based on this information, which three statements are plausible consequences of fracking in greendale?
Answers: 2

Biology, 21.06.2019 19:00
Consider the diagram below, which represents components of the biosphere. what do the two arrows in the diagram most likely represent? a. radiation b. photosynthesis c. cellular respiration d. energy conversions will give to anyone who answers quickly
Answers: 1


Biology, 22.06.2019 08:40
What best explains whether bromine (br) or neon (ne) is more likely to form a covalent bond? bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule. bromine forms covalent bonds because it has many electron shells, but neon has only two electron shells and is tightly bound to its electrons. neon forms covalent bonds because it can share its valence electrons, but bromine has seven valence electrons and can gain only one more electron. neon forms covalent bonds because it has only two electron shells, but bromine has many electron shells and will lose electrons in order to fulfill the octet rule.
Answers: 3
You know the right answer?
Questions

Social Studies, 14.01.2020 00:31

Health, 14.01.2020 00:31


Mathematics, 14.01.2020 00:31

Mathematics, 14.01.2020 00:31


History, 14.01.2020 00:31




Biology, 14.01.2020 00:31

History, 14.01.2020 00:31


Social Studies, 14.01.2020 00:31


Mathematics, 14.01.2020 00:31

Mathematics, 14.01.2020 00:31


Mathematics, 14.01.2020 00:31



