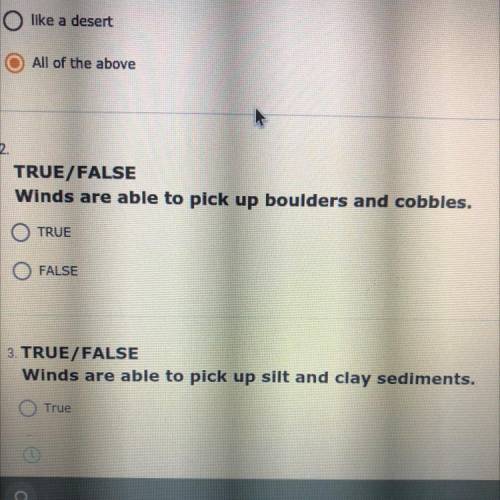
Uh does anyone know the answer?
...

Answers: 3


Another question on Biology


Biology, 21.06.2019 22:00
An ecologist is studying the effects that a population of predators is having on a population of a prey. he used data from the field to produce this graph. which conclusion can draw from the graph?
Answers: 3

Biology, 22.06.2019 00:00
Briefly describe the scenario that was discussed in the behaving brain that demonstrated that the brain can change based on state or mood. which disease could the research involving rats and learning experiments that was discussed in the behaving brain ultimately with? in the behaving brain, what is a common misunderstanding about amnesia that was mentioned? what is the truth about amnesia that's mentioned? what does research discussed in the responsive brain show to be different in men and women's reaction to touch? what was discussed in the responsive brain as being brain-based and a vital part of normal growth and development in young children? in the responsive brain, what do they discuss as a possibility for leading to deficiencies in cognitive impairment as we age? in the responsive brain, what did the research involving african cichlid fish demonstrate? briefly describe the back story and affliction of the author of peter pan. what did you find most interesting about the behaving brain video, and why? what did you find most interesting about the responsive brain video, and w
Answers: 3

Biology, 22.06.2019 08:40
What best explains whether bromine (br) or neon (ne) is more likely to form a covalent bond? bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule. bromine forms covalent bonds because it has many electron shells, but neon has only two electron shells and is tightly bound to its electrons. neon forms covalent bonds because it can share its valence electrons, but bromine has seven valence electrons and can gain only one more electron. neon forms covalent bonds because it has only two electron shells, but bromine has many electron shells and will lose electrons in order to fulfill the octet rule.
Answers: 3
You know the right answer?
Questions

Mathematics, 14.12.2019 20:31








History, 14.12.2019 20:31


Social Studies, 14.12.2019 20:31

Biology, 14.12.2019 20:31

English, 14.12.2019 20:31

Mathematics, 14.12.2019 20:31



Mathematics, 14.12.2019 20:31


English, 14.12.2019 20:31

Mathematics, 14.12.2019 20:31