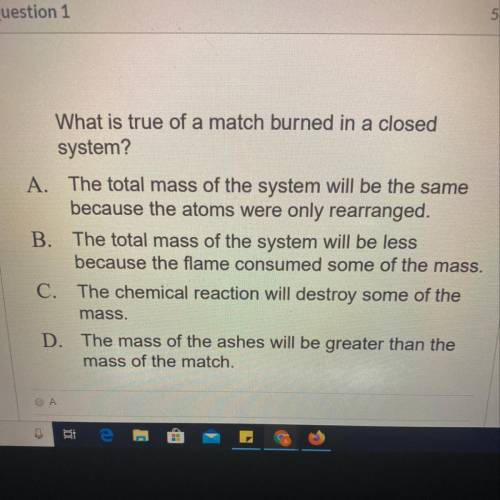
What is true of a match burned in a closed
system?
A. The total mass of the system...

Biology, 28.03.2020 00:18 paulinahunl17
What is true of a match burned in a closed
system?
A. The total mass of the system will be the same because the atmos were only rearranged.
B. The total mass of the system will be less because the flame consumed some of the mass.
C. The chemical reaction will destroy some of the mass.
D. The mass of the ashes will be greater than the mass of the match.

Answers: 2


Another question on Biology

Biology, 22.06.2019 00:00
Hurry which of these is true about index fossils? a) are very scarcely found b) used as guides in relative dating c) found in the youngest layer of the rock d) used as reference points in absolute dating
Answers: 2

Biology, 22.06.2019 13:30
What kinds of molecules can pass through the cell membrane without any problem?
Answers: 1

Biology, 22.06.2019 17:00
Some species of wasps are social. the queen starts a colony from scratch each spring. she builds a small nest, and lays and raises a group of female workers. the workers enlarge the nest while the queen continues to lay eggs. unfertilized eggs become males that mate with newly hatched females. all of the wasps except the newly fertilized females die by the summer. which best describes this behavior? a)it is beneficial only to the males that do not fertilize eggs. b)it is beneficial only to the female workers that are not fertilized. c)it is beneficial to each one of the individual colony members. d)it is beneficial to the whole species, but not to all of the individual members.
Answers: 1

Biology, 22.06.2019 17:30
The krebs cycle is also known as the calvin cycle pyruvic acid cycle carbon - oxygen cycle citric acid cycle
Answers: 2
You know the right answer?
Questions

Physics, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00


History, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00

History, 28.06.2019 10:00




Mathematics, 28.06.2019 10:00


Mathematics, 28.06.2019 10:00


History, 28.06.2019 10:00

History, 28.06.2019 10:00

Mathematics, 28.06.2019 10:00



Mathematics, 28.06.2019 10:00



