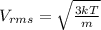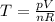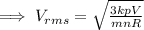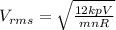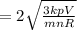Biology, 11.12.2019 04:31 dinarussell74
An ideal gas is held in a container of volume v at pressure p. the rms speed of a gas molecule under these conditions is v. if now the volume and pressure are changed to 2v and 2p, the rms speed of a molecule will be?
a) 4v
b) v/2
c) v/4
d) 2v
e) v

Answers: 1


Another question on Biology

Biology, 22.06.2019 03:00
What causes darkening of the skin as melanin production increases
Answers: 1

Biology, 22.06.2019 03:30
Identify any four organelles that should be present in the eukaryotic organism and describe the function of each organelle
Answers: 1

Biology, 22.06.2019 04:40
Awoman whose sister tested positive for a specific mutation in the brca1 gene, which increases the risk for breast and ovarian cancer, is found not to have that mutation but does have a mutation of unknown significance near the known mutation site. how should this woman be counseled? select one: a. she should be informed that her risk for breast cancer is greater than the general population but not as great as her sister’s risk. b. she should be informed that because she does not have the mutation, her risk for breast cancer is not greater than that of the general population. c. she should be informed of her gene mutation status and be presented with all the available prophylaxis options and reconstruction options. d. she should be informed that she does not have the specific mutation but that because another mutation is present she should be vigilant about screening
Answers: 1

Biology, 22.06.2019 05:00
Cedric has a low fever and minor aches. yesterday, he went to the doctor for his booster shot and receive the flu shot. which best explains why he has this reaction?
Answers: 2
You know the right answer?
An ideal gas is held in a container of volume v at pressure p. the rms speed of a gas molecule under...
Questions




Biology, 17.11.2020 18:50

Arts, 17.11.2020 18:50

Arts, 17.11.2020 18:50

Mathematics, 17.11.2020 18:50


Business, 17.11.2020 18:50


English, 17.11.2020 18:50

English, 17.11.2020 18:50

Mathematics, 17.11.2020 18:50

Spanish, 17.11.2020 18:50

Geography, 17.11.2020 18:50

Geography, 17.11.2020 18:50



Chemistry, 17.11.2020 18:50