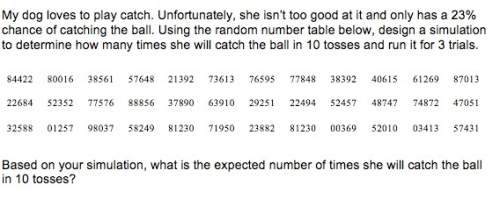
Advanced Placement (AP), 10.03.2021 03:50 lnc0500
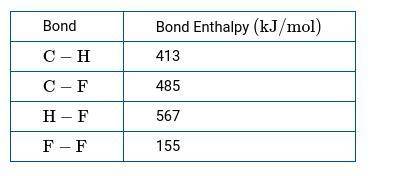
CH4(g) gas reacts with F2(g) to produce CH3F(g) and HF(g). b.) Use the bond enthalpies in the table below to calculate a numerical estimate of ΔH for the reaction.

Answers: 2


Another question on Advanced Placement (AP)

Advanced Placement (AP), 24.06.2019 16:30
Which of the following is a resource outside the workplace that will you find information on safety and health issues? a.safety data sheets b.medical manuals c.the osha website d.work task and procedures intruction
Answers: 1

Advanced Placement (AP), 25.06.2019 23:00
Which of the following best explains why the game of economics does not have a single goal
Answers: 1

Advanced Placement (AP), 26.06.2019 05:30
Treatment of psychological disorders: a. can offer significant relief b. often makes the problem worse. c.is often unclear d. is rarely .
Answers: 2

Advanced Placement (AP), 27.06.2019 07:30
How does using a credit card impact your spending habits?
Answers: 2
You know the right answer?
CH4(g) gas reacts with F2(g) to produce CH3F(g) and HF(g).
b.) Use the bond enthalpies in the table...
Questions

History, 17.11.2021 09:10

History, 17.11.2021 09:10



Biology, 17.11.2021 09:10



Mathematics, 17.11.2021 09:20



Mathematics, 17.11.2021 09:20

English, 17.11.2021 09:20

Chemistry, 17.11.2021 09:30

Computers and Technology, 17.11.2021 09:40

Mathematics, 17.11.2021 09:40



English, 17.11.2021 09:40

Social Studies, 17.11.2021 09:40