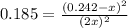Advanced Placement (AP), 26.02.2021 16:40 jdvazquez18p7a7vs
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.185. If 1.45 moles each of N 2(g)and O 2(g)are introduced in a container that has a volume of 6.00 liters and allowed to reach equilibrium at 300 K, what are the concentrations of N 2(g ) , O 2(g) ,and NO (g)at equilibrium?

Answers: 1


Another question on Advanced Placement (AP)

Advanced Placement (AP), 23.06.2019 19:00
Read the scenario below and answer the question that follows. bailey is plagued with sleep problems at least a couple of times a week. she often feels tired at work and doesn't do her best work because she is "just so tired." according to the lesson, it can be said that adults have some of the same problems with sleep that bailey does. a. few b. many c. no d. all
Answers: 1

Advanced Placement (AP), 23.06.2019 19:40
You want free points & brainliest? answer this drivers ed question correctly and i got you in which of the following situations should you use your vehicle's hazard lights? a. you're stopped on the side of the road with an engine that won't start. b. you're driving toward the shoulder because you hear a strange noise. c. you're driving in the rain and your headlights aren't functioning. d. you're driving slowly because you just spilled coffee on your lap.
Answers: 2

Advanced Placement (AP), 26.06.2019 20:30
Which area of the mind works based on the reality principle? id ego superego conscience
Answers: 1

Advanced Placement (AP), 28.06.2019 07:20
Analyze the economic development during the twentieth century in sub-saharan africa, southeast asia, or the middle east.(pick one region)#one paragraph is all i need
Answers: 1
You know the right answer?
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.18...
Questions








Biology, 18.01.2020 04:31


Mathematics, 18.01.2020 04:31

Social Studies, 18.01.2020 04:31


Biology, 18.01.2020 04:31

Mathematics, 18.01.2020 04:31


Biology, 18.01.2020 04:31

Mathematics, 18.01.2020 04:31



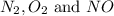 at equilibrium are 0.112 M, 0.112 M and 0.260 M
at equilibrium are 0.112 M, 0.112 M and 0.260 M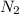 = 1.45 mole
= 1.45 mole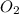 = 1.45 mole
= 1.45 mole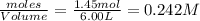
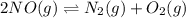
![K_c=\frac{[N_2]\times [O_2]}{[NO]^2}](/tpl/images/1150/8878/68b6f.png)