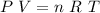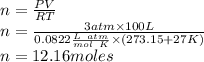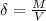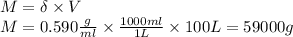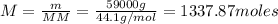Advanced Placement (AP), 28.11.2019 05:31 pr4ever
Propane, c3h8, liquefies under modest pressure, allowing a large amount to be stored in a container. calculate the number of moles of propane gas in a 100 −l container at 3.00 atm and 27 ∘c. calculate the number of moles of liquid propane that can be stored in the same volume if the density of the liquid is 0.590 g/ml.

Answers: 2


Another question on Advanced Placement (AP)

Advanced Placement (AP), 24.06.2019 12:30
How can symbols and absolute value you to order sets of integars
Answers: 1

Advanced Placement (AP), 24.06.2019 21:00
Exaggerated clams about a product appeared in advertising and on the product packages what are some examples of false promises made in marketing
Answers: 1

Advanced Placement (AP), 25.06.2019 04:30
Arrange the following in order from the shortest wavelength to the longest wavelength. visible light gamma rays radio waves x-ray waves microwave infrared
Answers: 1

Advanced Placement (AP), 25.06.2019 19:40
What are 3 traits that ruth may price has in the poisonwood bible? how has she adapted to the new situation? what effect has she had on others?
Answers: 2
You know the right answer?
Propane, c3h8, liquefies under modest pressure, allowing a large amount to be stored in a container....
Questions














Computers and Technology, 25.12.2020 17:30



Computers and Technology, 25.12.2020 17:30

Physics, 25.12.2020 17:30